
Nitrogen (N) is an element of great importance for life and the environment. Plants are fundamentally dependent on it, as it is the nutrient they need in the largest quantities. It is estimated that 85 – 90 million tons of nitrogen fertilisers are added to the soil worldwide each year. Nitrogen is a component of many plant cell compounds, including amino acids, hormones, nucleic acids and chlorophyll. Hence, it becomes understandable that nitrogen deficiency rapidly impedes plant growth and health. Nitrogen management, however, is a unique and complex process. Due to the peculiar forms of nitrogen in the soil, it has been estimated that 50 – 70% of the nitrogen applied to soil is lost. Therefore, improving the Nitrogen Use Efficiency (NUE) indicator is necessary to optimise crop yields and agricultural income, while minimising nitrogen losses to the environment.
1. Basic functions of nitrogen
Nitrogen is one of the most important nutrients for plants and it is considered a macro-element, as it is required in large quantities. From a physiological point of view, it is an essential component of nucleotides, amino acids, alkaloids, certain hormones, vitamins and chlorophyll molecules. It is, therefore, clear that it is a primary element for the processes of biosynthesis. Its nutritional adequacy contributes to the photosynthetic activity and the growth of plants, whose foliage presents a characteristic rich, dark green colour. In addition, there are several reports that nitrogen is positively correlated with plant resistance to diseases, such as botrytis and phytophthora. It also promotes the strengthening of the root system, the intake and availability of other nutrients, the increase of the plant’s protein content and the crop yield improvement.
Depending on the plant species, nitrogen accumulates in the vegetative parts of the plant at different concentrations during its biological cycle. The content of the shoots or stems of certain crops may be used as an indicator for the nitrogen status of the plants.
Both the reduction in production and the deterioration in quality are directly related to the intensity of nitrogen deficiency. In the event that the deficiency progresses, then the development of the underground and aboveground part of the plant is inhibited.
On the contrary, according to reports, excess nitrogen leads to a delay in maturation, greater susceptibility to enemies and diseases, and a deterioration in quality and production. In the case of cereals, excess nitrogen leads to severe wilting, due to the development of long internodes and pronounced tillering.
2. Nitrogen uptake
The forms of nitrogen that can be assimilated by plants are mainly nitrates (ΝΟ3–) and, secondly, ammonium (ΝΗ4+). Concerning the assimilation of nitrates (NO3–), initially ΝΟ3– nitrogen in the root system is converted to a higher energy form, ΝΟ2– nitrite nitrogen. This reduction is catalyzed by the enzyme nitrate reductase, the main component of which is molybdenum (Mo). This is the reason that one of the symptoms of molybdenum deficiency is the accumulation of nitrates, as a consequence of the reduced activity of the enzyme. Nitrite (ΝΟ2–) is a highly toxic and active ion, which must be removed immediately. It is, thus, converted into specific organelles, in an even higher energy form, to ammonium (NH4 +) (through nitrate reductase) and, finally, to amide nitrogen, into the amino acid glutamine (through glutamate synthase).
In most plants, nitrates (ΝΟ3–) are mainly reduced to the root system when it absorbs relatively small amounts of nitrates. The higher the nitrate intake, the greater is the percentage of nitrate transferred to the shoots and assimilated there.
For the uptake of ammonium ions (NH4+), the involvement of various and equivalent transport systems is, also, necessary in an equally high energy-intensive reduction process.
3. The dynamics of nitrogen in soil
The behaviour of nitrogen in soil is a complex process and is determined by several physical, chemical and biological factors. Molecular nitrogen (N2) accounts for 79% of ambient air but is in its inert form. It can be used by organisms only after it has been bound to or combined with other elements, such as oxygen (O2) or hydrogen (H2). Atmospheric nitrogen fixation/capture in soil is achieved:
- Through nitrogen-fixing bacteria of the genus Rhizobium, which form symbiotic relationships with leguminous plants.
- Through the Haber process (Reaction of N2 and H2, under conditions of high temperature and pressure).
- Through nitrate (ΝΟ3–) precipitates from lightning.
At the same time, nitrogen can be found in the soil in organic form (in the form of proteins, free amino acids, etc.), which, as will be seen below, can, of course, be converted into ammonium ions (ΝΗ4+) and nitrates (ΝΟ3–) through nitrifying bacteria.
Generally, the main inorganic forms of nitrogen found in soil are ammonium ions (ΝΗ4+), nitrates (ΝΟ3–), and traces of nitrites (ΝΟ2–). Ammonium ions as cations can be adsorbed by soil colloids, evaporated into ammonia gas under alkaline conditions, nitrified or bound to microorganisms and plants. Nitrates as anions are repelled and remain in soluble form, thus they are easily flushed out. Nitrites are unstable forms and are rapidly oxidised to nitrate ions through the nitrification process.
It has been estimated that approximately 66-92% of ammonium ions (ΝΗ4+) are converted to nitrate ions (ΝΟ3–) within 4 weeks. This conversion is done through a biological process called nitrification. Nitrification is the process by which ammonia (ΝΗ3) and its ionised form, ammonium (ΝΗ4+), are converted to nitrate anions (ΝΟ3–) by means of autotrophic nitrifying soil bacteria. Nitrification is carried out in two steps:
The firststep concerns the oxidation of ammonium to nitrites through the involvement of nitrifying bacteria of the genus Nitrosomonas, according to the following reaction:
2ΝΗ4+ + 3Ο2 à 2ΝΟ2– + 2Η2Ο + 4Η+ + Energy
The secondstage concerns the oxidation of nitrites to nitrates from bacteria of the genus Nitrobacter, according to the following reaction:
2NO2– + O2 à 2NO3– + Energy
The process of denitrification (conversion of nitrates into gaseous forms of nitrogen) and the evaporation of ammonia are equally important nitrogen escape routes.
An organic compound used as a fertiliser is urea CO(NH2)2. This compound has a nitrogen content of 46% w/w, but it is not readily available to plants. Soil urea is converted to ammonium (ΝΗ4+) by an enzyme called urease. A significant percentage of ammonium ions will be converted directly into ammonia (NH3), which will be evaporated into the atmosphere as a volatile substance. It is estimated that this conversion takes place over 1 – 4 days, after urea is applied on the surface of the soil. The percentage of nitrogen that will be lost in the form of ammonia (NH3) depends on the activity of the enzyme urease, which is present under certain soil and climatic conditions.
As seen from the above, the necessity of finding new fertilisers with increased nitrogen efficiency (NUE, Nitrogen Use Efficiency) is great. Therefore, the creation of fertilisers with the addition of inhibitors aims to reduce nutrient losses in the field, as well as their supply in plants at the appropriate period and in the appropriate quantity. This will, also, increase the efficiency of fertilisers and reduce environmental pollution.
The term “slow-release fertilisers” or “stabilised fertilisers” refers to fertilisers that contain urease inhibitors and/or nitrification inhibitors.
Urease inhibitors based on thiophosphoric triamide (NBPT) lock the binding sites of the urease enzyme. In this way, they delay the conversion of urea to ammonium ions (NH4+) and, therefore, reduce the possibility of volatility of ammonia (NH3).
Nitrification inhibitors based on dicyandiamide (DCD) and DMPP slow down the nitrification process by delaying the oxidation of ammonium ions (ΝΗ4+) to nitrogen dioxide (ΝΟ2–) and, subsequently, nitrates (ΝΟ3–).
The innovative use of nitrification and urease inhibitors is one of the strategies for the enhancement of nitrogen efficiency (NUE) that we have for different crops. It offers advantages to the producer, as well as the crops and the environment.
On the one hand, the action of the urease inhibitor drastically reduces nitrogen losses to the atmosphere in the form of ammonia. On the other hand, the action of the nitrification inhibitor minimises nitrogen losses from soil due to nitrate (ΝΟ3–) flushing and nitrogen losses to air in the form of nitrogen oxides (ΝΟ, Ν2Ο).
Cultivation ensures continuous and complete supply of the plants with nitrogen fertilisation. In the case of urease inhibitors, agricultural products are characterised by a high protein content, while in the case of nitrification inhibitors the accumulation of nitrates in the fruits and leaves of plants is avoided.
Producers, on the other hand, are given the possibility to reduce applications during the biological cycle, thus, requiring fewer cultivation works and leading to reduced costs. In addition, due to higher yields and better quality of agricultural products, they provide higher profits and agricultural income.
4. Nitrogen deficiency
The morphological and physiological changes caused by the lack of nitrogen suggest that this nutrient plays a central role in the growth of higher plants.
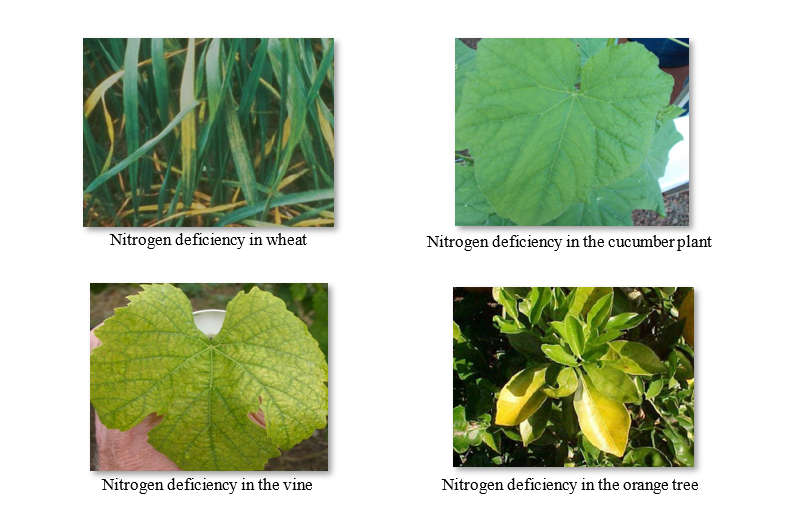
Nitrogen deficiency causes chlorosis (yellowing) of the leaf blade, especially of the older leaves near the base of the plant, since it is a mobile part. Chlorosis is due to the loss of nitrogen inside the chloroplasts.
When nitrogen deficiency progresses slowly, plants may have significantly thin and often lacerated shoots. This phenomenon can be attributed to the accumulation of excess carbohydrates, which cannot be used in the synthesis of amino acids or other nitrogen-containing compounds. The visible symptom is the purple coloration of the leaves, stems and shoots. This phenomenon is common in plants, such as tomatoes and certain varieties of maize.
According to several reports, when nitrogen is administered in its ammonium form (ΝΗ4+), interactions are observed with other inorganic nutrients, such as potassium (K), calcium (Ca), and magnesium (Mg); at high concentrations it can, also, lead to deficiency of the above elements. When nitrogen is applied in its nitrate form (ΝΟ3–), it competes with Sulphur (S) and phosphorus (P).
5. Nitrogen fertilisation solutions
Firstly, we suggest that you conduct a soil analysis of your field every two years, so that you have a complete picture of the nutrient levels. Compare your yield goals with the availability of your nutrients and discuss the available options with your agronomist. Due to the fine line between nitrogen deficiency and toxicity, it is important to apply the correct nitrogen fertiliser, at the right rate, always using the right product.

NEON® is a granular, controlled-release nitrogen fertiliser with a double inhibition, that of urease (NBPT) and that of nitrification (DCD), and uses PENXCEL technology for the optimisation of nitrogen fertilisation. The NEON granular fertiliser contains a urease inhibitor (NBPT) to prevent nitrogen losses in the form of ammonia gas and a nitrification inhibitor (DCD) to reduce nitrogen losses due to flushing. The increased penetration of the combination of the two inhibitors in the fertiliser granules, using PENXCEL technology, creates a double “safety net” for nitrogen.

SLOWTEC® is a granular, slow-release nitrogen fertiliser with a nitrification inhibitor (DMPP) for the optimisation of nitrogen fertilisation. The SLOWTEC series is based on the prevention of nitrification of ammoniacal nitrogen, resulting in the active avoidance of nitrate losses due to flushing and the continuous supply of nitrogen to the crop for an extended period of time.

UNIQUE® is a granular, controlled-release nitrogen fertiliser with a urea inhibitor (NBPT), using PENXCEL technology for the optimisation of nitrogen fertilisation. The UNIQUE series incorporates the technology of urease inhibitors. The application of Unique fertilisers reduces nitrogen losses in the form of ammonia gas and allows for more flexibility in the fertiliser’s period of application. The UNIQUE series includes fertiliser types ideal for basic fertilisation, but also types that meet the increased nitrogen requirements during the critical stage before and after flowering.

