
Iron (Fe) is the most abundant chemical element on our planet. For plant organisms, it is considered a very important element for the process of chlorophyll formation, photosynthesis, activation of specific metabolic pathways and respiration. Iron deficiency is a global crop issue, with a serious impact on yield and quality of the products harvested and, thereby, on agricultural income. Iron deficiency is mainly linked to high soil pH. Drupes, citrus fruits, kiwis, vines, rice, soybeans, tomatoes, cucumbers and strawberries are some of the crops that are very susceptible to iron deficiency.
1. Basic functions of Iron
Iron (Fe) has many important functions regarding the development of plant organisms. Its role in photosynthesis is crucial. In particular, it binds to the active part of an enzyme, which catalyses the precursor molecule of chlorophyll biosynthesis; therefore, its deficiency causes chlorosis. Iron is, also, an essential component of a protein (ferredoxin), which is required for nitrate reduction, sulfate reduction, nitrogen assimilation and energy production (NADP+). At the same time, it plays an important role in both DNA synthesis and ethylene synthesis. The indirect functions attributed to iron include the meristematic growth of the root tip.
2. Availability
Although most of the total iron in soil is trivalents (Fe3 +), divalent iron (Fe2+) is the main assimilable form for plants. This form is relatively soluble, but oxidizes fairly easily to Fe3+. Fe3+, however, is insoluble in a neutral and alkaline environment, making it unavailable to plants. In these soil types (limestone soils), the use of iron chelates (iron complexes with organic matter) is recommended. It is, therefore, understood that the solubility of iron depends, to a large extent, on the pH value.
The interaction of iron with other nutrients is, also, quite an important factor that affects its availability. High levels of Zinc (Zn), Copper (Cu), Phosphorus (P), Calcium (Ca), Boron (B) and Manganese (Mn) may interfere with the solubility and uptake of Iron (Fe) by plants. In contrast, Potassium (K) and Sulphur (S) appear to have a positive correlation with Iron. Nitrogen (N), by promoting germinal growth, also enhances the deficiency of iron (an element with low mobility).
Additional factors affecting the availability of Iron are the percentage of organic matter, temperature, soil moisture, amount of rainfall and porosity.
3. Intake
Plant organisms, in their effort to combat iron deficiency, have developed various intake strategies:
- Strategy I: The root system releases substances, which reduce the value of soil pH. This category includes legumes.
- Strategy II: secretion of chelating agents (phytosiderophores, iron-carrier compounds), which form complexes with iron, resulting in increased solubility and intake. Cereals belong to this category.
Also, the selection of nitrogen fertiliser significantly affects iron intake. Ammoniacal fertilisers that reduce pH favour iron intake. On the other hand, nitrates make iron intake more difficult, because they increase pH.
4. Iron defeciency
The symptoms of Iron (Fe) deficiency are similar to those of magnesium (Mg) deficiency, because both elements are involved in chlorophyll biosynthesis. However, because iron is not a mobile element within the plant (while magnesium is mobile), the symptoms of iron deficiency first appear in younger leaves. In general, iron deficiency occurs when iron concentration falls below 10 – 30 ppm. In conditions of deficiency, the leaf blade shows interveinal chlorosis, the growth of the top meristems is reduced, the chlorosis extends to the remaining leaves and there is defoliation.
Fe deficiency is the most common trace element deficiency in a wide range of crops. Iron deficiency is pronounced in drupes, citrus fruits, kiwifruits, vines, rice, soybeans, tomatoes, cucumbers and strawberries. Some examples of iron deficiency in certain crops can be found below.
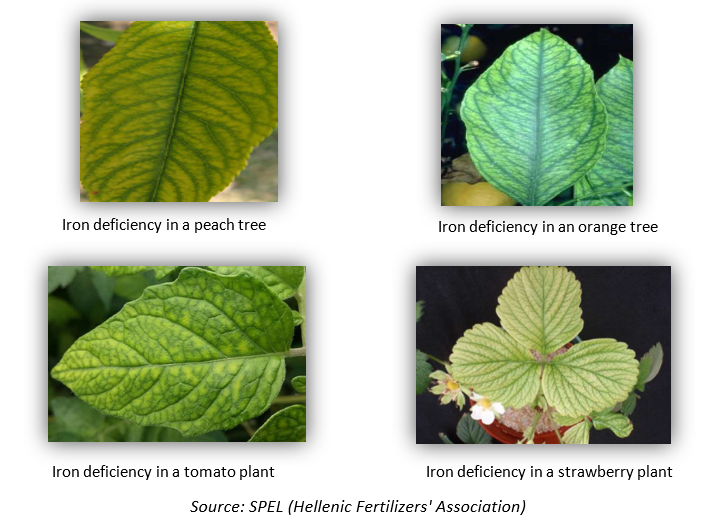
5. Tips for preventing iron deficiency
Firstly, we suggest that you conduct a soil analysis of your field every two years, so that you have a complete picture of the nutrient levels. Compare your yield goals with the availability of your nutrients and discuss the available options with your agronomist. It is important to apply the right amount of iron, at the right rate, always using the right product, as there is a fine line between nutrient deficiency and toxicity.
Depending on the soil conditions of each field, iron fertilisers are classified in the following categories:
- Iron sulphate (Fe2SO4): This type of fertiliser contains on average 20% iron and is suitable for application on acidic soils. It is mainly applied through the leaves.
- Iron chelates:
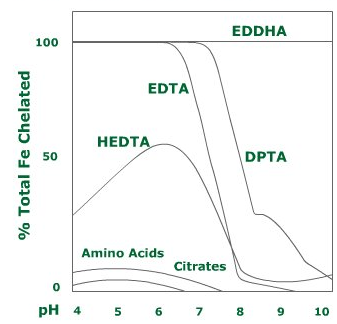
- Fe – EDTA: This type of iron fertiliser is stable in soils with a pH value below 6,5. If the pH rises by even half a unit, almost 50% of the iron will not be available.
- Fe – DTPA: This iron chelate is stable up to a pH of 7,5.
- Fe – EDDHA: This type of fertiliser is stable over the whole pH range (up to 12).
Our company has a complete range of iron nutrition products.

FERRI – GREEN® is a high quality, solid iron chelate. It contains 6% chelated iron (Fe) EDDHA, at a high ortho-ortho isomer ratio (80%). It is a fertiliser that remains stable over the whole pH range (from 3 to 12). It can be applied to all crops, including vegetables, vines, strawberries, kiwifruits, drupes and citrus fruits. The typical recommended dose is 5 – 15 kg / stremma. FERRI – TOP® is a similar product that our company offers. More information about the detailed product use guide can be found in the “Products” tab on our website.
The Fe – DTPA® chelated fertilisers offer optimum efficiency, as they are adapted to the needs of hydroponics. Fe – DTPA® 11% powder is a solid fertiliser which is stable at pH values from 3 to 8. The recommended dose for foliar applications is 500 – 1000 gr / tn. Fe – DTPA® 6% liquid is a liquid fertiliser which is stable at pH values from 3 to 8. The recommended dose is 3 L/tn. More information about the detailed product use guide can be found in the “Products” tab on our website.
VIOMIX AMINO – Fe® is a liquid iron chelate that is different from the others. Instead of an organic complex, this fertiliser contains an iron complex with 5% L – amino acids. It can be applied for a number of crops and the recommended dose through foliar application is 3 L/tn.

Fe – ELEMENTS® is a specialised product of 13.2 % Iron (Fe) EDTA in solid form. It is 100% water-soluble and contains iron chelate EDTA (Fe), so as to ensure maximum intake of the element during its application. It is suitable for both foliar applications and fertigation. It is a fertiliser which remains stable at pH values from 3 to 7. More information about the detailed product use guide can be found in the “Products” tab on our website.

